Master Bond’s Epoxy Compatibility with STERIS’s Vaporized Hydrogen Peroxide Sterilization Process
As medical devices have evolved through technological advancements, they have become more complex in both their design and materials of construction. Medical grade biocompatible epoxies are widely used in reusable medical devices. Choosing an epoxy that maintains its performance characteristics when subjected to repeated sterilization throughout the reusable medical device’s lifespan is a known challenge for medical device manufacturers.
Master Bond and STERIS collaborated in research1 to determine the compatibility of Master Bond’s specialty epoxies with Vaporized Hydrogen Peroxide (VHP™) using V-PRO® s2 Low Temperature Sterilization System. This study was recently published in a peer-reviewed journal Polymer-Plastics Technology and Materials.
As medical devices have evolved through technological advancements, they have become more complex in both their design and materials of construction. Medical grade biocompatible epoxies are widely used in reusable medical devices. Choosing an epoxy that maintains its performance characteristics when subjected to repeated sterilization throughout the reusable medical device’s lifespan is a known challenge for medical device manufacturers. This study evaluated the material compatibility of seven cured two part and one part epoxies used in medical devices following exposure to 100 cycles in a low temperature vaporized hydrogen peroxide sterilizer.
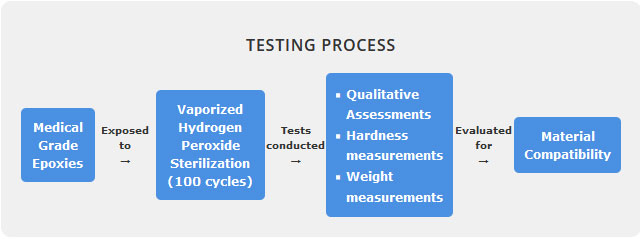
Six of the seven epoxies tested were found to be compatible with vaporized hydrogen peroxide sterilization based on qualitative, hardness and weight measurements conducted post exposure to 100 VHP cycles. The epoxies deemed to be compatible displayed no visual signs of physical defects, minimal reduction in hardness (≤2%) and total weight gain (≤2.9%). This study highlights the importance of conducting material compatibility studies, and continued collaborations (among medical device manufacturers, sterilizer manufacturers and epoxy manufacturers) during the early stages of medical device development to ensure a successful reusable medical device that will withstand repeated sterilization.
Key Findings from the Study about Master Bond Medical Adhesive Systems
The Master Bond EP42HT-2Med, EP42HT-4AOMed Black, EP62-1HTMed, EP41S-5Med, EP4CL-80Med and UV10TKMed epoxies are compatible with low temperature vaporized hydrogen peroxide, as they maintained their surface texture, exhibited less than 2% change in hardness, and displayed less than a 2.5% weight gain post exposure to 100 VHP cycles.
To read the full article online please visit https://www.tandfonline.com/doi/full/10.1080/25740881.2024.2376209#abstract, or download the article here.
Source
1Shruti Padhee, Christine Fingar, Ankit Patel, Randal Eveland, Jordan Rantucci, Tawana Ward, Venkat Nandivada & Rohit Ramnath (21 Jul 2024): Material compatibility of epoxies exposed to repeated low temperature vaporized hydrogen peroxide sterilization. Polymer-Plastics Technology and Materials, DOI: 10.1080/25740881.2024.2376209
Epoxies Tested in the Study
 |
EP42HT-2Med Low viscosity, two part epoxy with outstanding chemical resistance. Passes USP Class VI biocompatibility tests. Capable of withstanding repeated sterilization cycles including radiation, EtO, chemical sterilants, autoclaving. Serviceable from -60°F to +450°F. Cures at room or elevated temperatures. Available in amber-clear and black colors. Castable in thicknesses up to 2-3 inches. |
 |
EP42HT-4AOMed Black Thermally conductive, electrically insulative two part epoxy. Meets USP Class VI and ISO 10993-5 certifications. Cryogenically serviceable from 4K to +400F. NASA low outgassing aproved. Black in color. For bonding, sealing, coating and casting. |
 |
EP62-1HTMed Superior sterilization resistance. Two part epoxy has long pot life at ambient temperatures. High bond strength properties. Ideal for bonding and coating. Good flow. Reliable electrical insulator. Serviceable from -60°F to +450°F. Tg 150-155°C. Shore D hardness 80-90. |
 |
EP41S-5Med Excellent chemical resistance to solvents, bases, acids, alcohol and fuels. Withstands exposure to methylene chloride, phenol (10%) and nitric acid (30%). Well suited for coating tanks, pumps and vessels. Moderate viscosity with good flow properties. Can be used for potting/encapsulation. Formidable physical strength properties. Serviceable from -80°F to +450°F. |
 |
EP4CL-80Med One component, ultra low viscosity epoxy that offers thermal and electrical insulation and is optically clear. |
 |
UV10TKMed Biocompatible, high viscosity, UV curable compound. Optically clear. Glass transition temperature of more than 140°C. Rapid curing, no mix system. Withstands repeated sterilization. Meets USP Class VI and ISO 10993-5 cytotoxicity testing requirements. Serviceable from -60°F to +450°F. |
