
In the adhesive vocabulary, the term “wetting” refers to the ease with which an adhesive can intimately contact and spread over a given substrate. There are a variety of forces (ionic, static, polar, van der Waals etc.) acting between the adhesive and the substrates that ensure good bonding. Good wetting provides a larger area of contact where these forces may act. Consequently, good wetting is crucial for good bond formation.
The degree of wetting of any given substrate can be attributed to the surface tension or the critical surface energy of the substrate and the adhesive. As a general rule, for good wetting, the critical surface energy of the substrate should be higher than the critical surface energy of the adhesive.
A substrate with higher critical surface energy is wetted-out more readily as compared to a substrate with a lower energy. For example, metals like Aluminum or Copper have a higher critical surface energy and wet more easily as compared to substrates like Polyethylene or PTFE. The surface energy of a standard epoxy is approximately 40-50 mJ/m2. The table below provides the critical surface energy values for some of the most widely used substrates:
| Substrate | Critical Surface Energy mJ/m2 |
| Aluminum | ~500 |
| Copper | ~1,000 |
| Glass | ~1,000 |
| Polycarbonate | 46 |
| PTFE | 18 |
| Polyethylene | 31 |
A good way to assess whether a surface can be wetted effectively or not is by spraying distilled water over the surface and measuring the contact angle between the water bead and the surface. The relation between the contact angle and the surface wettability is illustrated in the figure below.
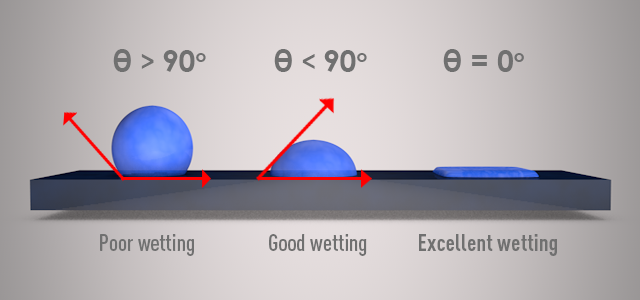 The low critical surface energy of epoxies makes them a distinguished choice for use as adhesives for a variety of substrates. Master Bond custom formulates an array of adhesives that offer excellent adhesion, wetting and physical strength for bonding a number of similar and dissimilar substrates. Additionally, good surface preparation helps improving the wettability of a substrate, which in turn improves the bond strength.
The low critical surface energy of epoxies makes them a distinguished choice for use as adhesives for a variety of substrates. Master Bond custom formulates an array of adhesives that offer excellent adhesion, wetting and physical strength for bonding a number of similar and dissimilar substrates. Additionally, good surface preparation helps improving the wettability of a substrate, which in turn improves the bond strength.
